Singapore regulations on healthcare products
Introduction
Regulating healthcare products is an integral part of the regulatory framework in most developed countries. These regulations are designed to reduce potential health risks to citizens and to ensure access to high-quality, effective, safe health products. At the same time, regulations help restrict access to products that may be ineffective or even harmful. Properly implemented laws applied to such products lead to better public health.
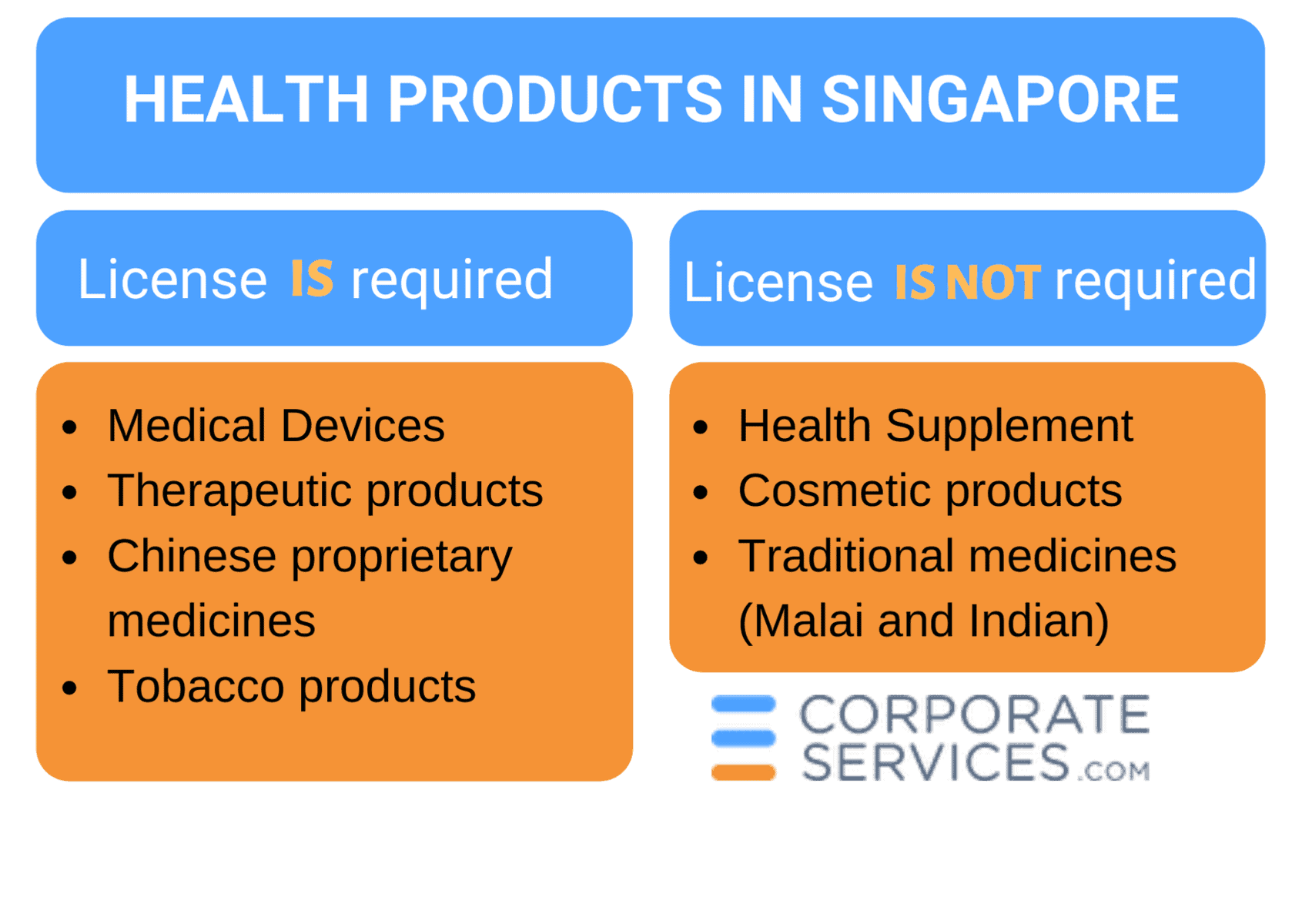
In Singapore, the term healthcare products applies to medical devices, therapeutic products, health supplements, traditional medicines, and cosmetic products. Tobacco products are also covered by these regulations.
Regulations apply to all phases of a health product’s life span, including:
- Pre-market stage: Public description of the health product, its classification, efficacy, quality and safety standards, etc.;
- Placement on the market: Registration process, listing of health products, licensing, import controls, etc.; and
- Post-market regulation: Adverse-event reporting, post-market surveillance, labeling requirements, and advertising.
Below, we will review the basic health product regulations in Singapore that companies must follow when manufacturing, importing, and selling healthcare products.
This article includes the following topics:
Why is Singapore’s healthcare market booming?
Singapore serves as the healthcare and medical hub of Southeast Asia. Singapore's healthcare system is ranked 6th in the world and offers the 4th best healthcare infrastructure globally.
Consequently, the healthcare market is booming in Singapore (S$21.4 billion in 2020) and is expected to more than double by 2029. There are several reasons for this:
- Government spending on healthcare is rising (S$13.2 billion in 2020, and S$36 billion is expected by 2029).
- Singapore is a hub for re-exported healthcare products, as more than 75% of products imported are subsequently re-exported to other countries.
- Some of the world's best hospitals, such as Singapore General Hospital, and prominent international healthcare and research organizations such as the American Association for Cancer Research, Duke University, and the Healthcare Information and Management Systems Society are established in Singapore. These hospitals often serve clients from outside Singapore as well.
- Singapore has one of the world’s most rapidly aging populations.
- In Singapore, a trend toward earlier diagnosis of chronic diseases means more time is spent on close monitoring and follow-up.
- Importation of equipment and supplies to Singapore has increased over the past decade, approximately by 6% per year.
- The city-state has one of the most pro-business environments in the world
- Singapore is developing sensible legislation in the healthcare products sector that strikes a good balance between the city-state’s priorities and the needs of businesses.
Below we will review key regulations that may be helpful for entrepreneurs who wish to start their venture in the healthcare products industry in Singapore.
Thinking of registering a company in Singapore?
Let's get started!
EASIER, FASTER, BETTER
Key Singapore laws for healthcare-related products
The main legislation governing healthcare products in Singapore includes the following:
- Health Products Act;
- Medicines Act;
- Medicines (Advertisement and Sale) Act;
- Sale of Drugs Act;
- Poisons Act; and
- Tobacco (Control of Advertisement and Sale) Act.
These laws establish a regulatory regime for all types of health products.
The Singapore government regularly reviews and updates legislation in this field. The latest changes were introduced at the end of 2020. Currently, issues concerning cell, tissue, and gene therapy products under the Health Products Act are under public consultation.
Who regulates Singapore’s healthcare sector?
The national regulatory authority responsible for supervising the healthcare products industry in Singapore is the Health Sciences Authority (HSA). It is a multi-disciplinary agency, but the division responsible for enforcement of laws in this sector is the Health Products Regulations Group.
The Health Products Regulation Group comprises the following clusters:
- Pre-market Cluster;
- Vigilance, Compliance & Enforcement Cluster (including audit and licensing & certification); and
- Medical Devices Cluster.
This Group ensures that the healthcare products available in Singapore meet standards of quality, efficacy, and safety. The HSA is also responsible for post-market activities such as adverse-effect reporting and surveillance.
What types of healthcare products are regulated in Singapore?
1. Medical Devices (MD)
The definition of a particular healthcare product guides manufacturers and importers as to the scope of the regulated product.
In general, Medical Devices (MD) in Singapore are health products that have a physical or mechanical effect on human bodies. Mainly, such products are used for:
- Diagnosis, alleviation, and treatment of a medical condition (contact lenses, wheelchairs, X-ray machines, etc.);
- Measuring or monitoring functions of the human body (blood sugar monitoring machines, pregnancy self-testing, etc.);
- Supporting or sustaining life (heart valves, implantable defibrillators, etc.);
- In vitro diagnostics.
Singapore has defined risk classifications for medical devices, which determine registration and licensing requirements. There are four risk classes:
- Class A — low-risk devices (surgical masks, tongue depressors, bandages, etc.);
- Class B — low to moderate risk devices (contact lenses, dental crowns, hearing aids, etc.);
- Class C — moderate to high-risk devices (hip implants, X-ray machines, lung ventilators, etc.);
- Class D — high-risk devices (breast implants, heart stents, heart valves, etc.).
Key facts you should know:
- Registrant must determine the risk class by himself via the on-line medical device risk classification tool.
- No special permit is needed to advertise medical devices. The advertiser is responsible to ensure compliance with the legislation and guidelines for advertisement and promotion of MD.
- All information on a label of MD must be in English.
- There is post-market control of MD:
- Every manufacturer, importer, supplier, or registrant of MD must maintain a record of every received complaint;
- Every manufacturer, importer, supplier, or registrant of MD must report any adverse event to the HSA.
2. Therapeutic products (drugs)
Therapeutic products, also known as drugs or pharmaceuticals, are healthcare products for therapeutic, preventive, palliative, or diagnostic purposes. All drugs require registration and licensing by the HSA before they can be imported, manufactured, or sold wholesale in Singapore.
Key facts you should know:
- Clinical trials are required for drugs in accordance with the HSA regulations.
- No special permit is required to advertise therapeutic products.
- It is mandatory for companies to report all serious adverse events regarding drugs.
3. Health Supplement
In Singapore, health supplements belong to the group of complementary health products that are regulated as healthcare products. A health supplement is a product used to supplement a diet with benefits beyond those of normal nutrients, and to support or maintain the healthy functions of the human body.
Typically, a health supplement consists of vitamins, minerals, amino acids, and botanical materials in the forms of extracts, isolates, or concentrates. It may be produced in capsules, soft gels, tablets, syrups, or liquids. Products presented in the form of food and beverages are not health supplements.
Key facts you should know:
- Health supplements are not subject to approvals and licensing by the HSA for their importation, manufacture, and sales. However, importers, manufacturers, or wholesalers must ensure that their products are not harmful or unsafe.
- There is post-market surveillance to monitor the safety of health supplements and to initiate timely product recalls.
- There are several prohibitions concerning the addition of medicinal ingredients such as steroids in health supplements.
- There are strict labeling standards for health supplements.
4. Cosmetic products
A cosmetic product is defined as any substance or preparation intended to be placed in contact with various external parts of the human body with a view to cleaning or perfuming them, changing their appearance, protecting them, or just keeping them in good condition.
Injections and eye drops, as well as products for treatment (e.g. creams against acne) are excluded from the cosmetic definition and are considered therapeutic products.
Key facts you should know:
- In Singapore, cosmetic product manufacturers are not required to apply for a special licence. However, the manufacturer may apply for a Good Manufacturing Practice certificate to facilitate the export of its cosmetic products.
- Labels or labeling statements must be in English and legible. All cosmetic products must comply with label requirements.
- Singapore's regulations on the quality and safety of cosmetic products are similar to the requirements for such items in the European Union.
- The company or person responsible for placing a cosmetic product in the market must notify the HSA of the place where the product will be marketed, of the place of manufacture, or of initial importation before the product is placed in the Singapore market.
5. Traditional Medicines (TM)
In Singapore, traditional medicines cover Indian and Malai traditional medicinal products. These products belong to a complementary health product group and are also regulated by the healthcare products regulations.
Key facts you should know:
- Traditional medicines are not subject to approvals and licensing by the HSA for their importation, manufacture, and sales. However, importers, manufacturers, or wholesalers must ensure that their products are not harmful or unsafe.
- To ensure that each production batch of TM is not contaminated with high levels of heavy metals, dealers should conduct heavy metals testing for every production batch.
- There are strict labeling requirements for TM marketed in Singapore.
6. Chinese Proprietary Medicines (CPM)
Chinese proprietary medicines are considered to be complementary health products in Singapore. CPM refers to medicinal products that contain ingredients used in traditional Chinese medicine. CPM can be produced in the form of a finished product, i.e. tablet, capsule, etc., but not in the form of injection.
Key facts you should know:
- A special permit from the HSA is required to advertise CPM or conduct any sales promotion activities.
- Labeling standards differ from those applicable to the other types of health products.
- Companies must report all serious adverse events.
7. Tobacco products
In Singapore, tobacco products are regulated by health products regulations. Tobacco products refers to cigarettes, shisha (waterpipe), cigars, and any product containing tobacco.
Key facts you should know:
- Imitations of tobacco products, such as electronic cigarettes, are prohibited by law.
- Chewing tobacco is also prohibited in Singapore.
- Advertisements and promotions relating to tobacco products and imitating tobacco products are prohibited.
- There are only six health warning labels that can be placed on tobacco product packages.
Which license should you obtain, if any?
In Singapore, healthcare products may be divided into two groups: those that require registration and licensing and those not subject to these requirements. In this section, the licensing requirements for each healthcare product category are described.
Medical Devices (MD)
Before obtaining any kind of license in Singapore, medical devices must be registered with the Medical Device Information Communication System (MEDICS).
Class A medical devices are exempted from product registration (see classification above). For B, C, and D groups, registration is done through an evaluation process. The period of time for this process and registration fees depend on the number of prior approvals given by overseas reference regulatory agencies, risk classification of the MD, and the length of the company’s safe marketing history.
After registration, it is possible to apply for a medical device dealer's license, which covers manufacturer, importer, and wholesaler’s licenses.
Types of licenses that may be required:
- Manufacturer's license is required for companies to
- Process, produce, or make any medical devices;
- Label and package MD before it is supplied.
Companies that obtain a manufacturer's license do not need a wholesaler's license to supply MD produced under their manufacturer's license.
- Wholesaler's license is required for companies to
- Supply MD by wholesale in Singapore (including for export);
- Supply MD to another party for re-supply.
Companies that supply MD to end-users do not need the wholesaler's license.
- Importer's license is required for companies to
- Import MD into Singapore.
Licenses of all types are valid for twelve months and then must be renewed.
Chinese Proprietary Medicines (CPM)
To manufacture, assemble, wholesale, or import CPM in Singapore, a product listing approval and a dealer's license are required. Before applying for a license, a company must also obtain product listing approvals from the HSA for the Chinese proprietary medicines that it produces.
Types of license that may be required:
- Manufacturer's license is required for a company to manufacture or assemble CPM. Companies that have this license do not require a wholesale dealer's license to wholesale the CPM.
- Wholesaler's license is required for companies to supply CPM by wholesale in Singapore. However, if a company wholesales CPM solely for use as clinical research materials in clinical trials, a license is not required.
- Importer's license should be obtained by companies that wish to import CPM into Singapore. However, companies that are importing CPM solely to export or re-export to another country do not need to apply for an import license for CPM.
In addition, as some importing countries may require a certificate to show that CPM is approved for sale and distribution in Singapore, companies may apply solely for a Certificate for Exporter of CPM (Free Sale Certificate), which is issued only for 1 product and allows the inclusion of up to five importing countries.
Tobacco products
To import, wholesale, distribute, or retail any tobacco product in Singapore, a tobacco license is required.
Types of license:
- Tobacco retail license is necessary to start retail sales of tobacco products. This kind of license is issued solely to a particular outlet. Licenses are non-transferable between outlets.
- Import and wholesale license is required for companies to import and wholesale tobacco products in Singapore. To obtain this license, companies must comply with the rules and regulations of the Tobacco (Control of Advertisements and Sale) Act.
Therapeutic products
Before obtaining any kind of license in Singapore, therapeutic products must be registered. Registration requirements will differ depending on whether companies submit a new drug application or generic drug application. After registration, it is possible to apply for a therapeutic product dealer's license.
Types of license that may be required:
- Therapeutic Product Manufacturer’s License (TPML) is required to manufacture or assemble drugs. Companies that hold a TPML do not need a Therapeutic Product Importer's License (TPIL) or Wholesaler's License (TPWL) for products manufactured in accordance with the company's TPML.
- Form A Poisons License (FAPL) is required to import and store products necessary for the manufacture of licensed therapeutic products. Companies that obtain a TPML do not need this license.
- Therapeutic Products Importer’s License (TPIL) is required to import therapeutic products.
- Therapeutic Products Wholesaler’s License (TPWL) is required to wholesale drugs in Singapore.
Companies do not need to obtain an importer's license (TPIL) or a wholesaler's license (TPWL) for therapeutic products for use as clinical research materials in clinical trials only.
| Type of Product | License Applicable | Requirement |
|---|---|---|
| Medical Devices (MD) | Manufacturer's license | Required for produce, label, and package of MD |
| Wholesaler's license | Required for wholesale and re-supply of MD | |
| Importer's license | Required for import of MD | |
| Therapeutic Products (ThP) | Therapeutic Product Manufacturer’s License (TPML) | Required |
| Form A Poisons License (FAPL) | Required for import and storage of products required to manufacture ThP | |
| Therapeutic Products Importer’s License (TPIL) | Required for import of ThP | |
| Therapeutic Products Wholesaler’s License (TPWL) | Required for wholesale | |
| Chinese Proprietary Medicines (CPM) | Manufacturer's license | Required for assemble or manufacture of CPM |
| Wholesaler's license | Required for wholesale | |
| Importer's license | Required for import of CPM | |
| Tobacco Products (TP) | Tobacco retail license | Required for retail sales of TP |
| Import and wholesale license | Required for import and wholesale |
Conclusions
Well-designed and reasonable regulations on healthcare products are a key factor of the flourishing healthcare market in Singapore. The government regularly reviews and updates legislation in this field, so as to establish a balanced regulatory regime that meets the requirements of a business-friendly environment as well as quality and safety standards.
Please contact CorporateServices.com team if you plan to apply for any license related to healthcare products activities or are still thinking about incorporating your Singapore venture. We can provide complete support for executing your corporate plans and help you with any other business regulatory issues in Singapore.
Eddy and Hannah
Your Customer Service Team
Work with a team that reflects Singapore's tradition of excellence in diversity. We speak many languages, come from different backgrounds, but we share one goal — your success in Singapore!
Tiffany
Nadia
Tonya
Fenni
Elena
Haly
Sandy
Mithun
Qid
Vika
Patricia
Benny
Jessica
Lingna
